Glucose Transporter Type 1 Deficiency Syndrome (Gluti) and Using Ketogenic Diet in Treatment of de Vivo Disease -Juniper Publishers
Global Journal of Intellectual & Developmental Disabilities (GJIDD)
We present experience of ketogenic diet (KD) applying
in the treatment of pharmacoresistant epilepsy in a patients with
glucose transporter deficiency syndrome type I (GLUT1). We observed six
children with refractory epilepsy due to GLUT1. The high effectiveness
of KD in the treatment of GLUT1 was demonstrated. All patients were
achieved complete absence of seizures and EEG abnormalities from the
beginning of KD. We noticed positive shift in cognitive and speech
development for all children. Antiepileptic drugs were stopped taking
due to the stable remission. There was a further positive dynamics in
intelligence, psycho-emotional sphere; the children began to go a
nursery school and a special school. Thus, the ketogenic diet is high
effectiveness and, perhaps, the only method for GLUT1 treatment.
Keywords: Ketogenic diet; Pharmacoresistant epilepsy; Intractable epilepsy; Glucose transporter deficiency syndrome type I (GLUT1)Introduction
Glucose transporter type 1 deficiency syndrome
(GLUT1) (synonyms: Glutl-DS, G1D, or De Vivo disease) is a rare genetic
disorder mainly affecting the CNS. The disease was first described in
1991. About 500 patients have now been recorded around the world. The
incidence ofGLUTl is independent of gender, age, and nationality [1,2]
The disease is caused by a defect in the SLC2A1 gene,
which encodes the glucose transporter responsible for transporting
glucose from the blood to the brain across the blood-brain barrier -
GLUT1 (type 1 glucose transporter). Mutations in the SLC2A1 gene can
alter or completely block the function of GLUT1 protein, with the result
that the brain lacks its main energy substrate, glucose, leading to
progressive impairment to brain functions and the occurrence of the
corresponding symptomatology
The level of cerebral glucose metabolism is low
during intrauterine development, increases linearly after birth, and
reaches a peak at age three years, after which it remains high
throughout the first decade of life, then gradually decreasing during
the second decade of life. Thus, it can be suggested that the risk of
clinical manifestations of GLUT1during intrauterine development is low,
but then increases during infancy and early childhood.
The genetic aspects of GLUT1 include mutations in the
SLC2A1 gene, generally spontaneous, though some families have been
described as having autosomal dominant inheritance. GLUT1 is on rare
occasions inherited as an autosomal recessive. The severity of the state
is determined by the characteristics of the mutation. Prenatal
diagnosis can be performed in high-risk pregnancies [3].
Children with GLUT1 have no phenotypic features at
birth. The disease subsequently develops in two variants: the classical,
or epileptic (90% of patients), and the non-epileptic (in 10% of
patients). The classical variant typically manifests in the first months
of life as polymorphous epileptic seizures: generalized tonic-clonic,
myoclonic, atypical absence, atonic and myoclonic-atonic seizures.
Seizures can occur with different frequencies - from monthly to daily,
and are characterized by marked resistance to anticonvulsant therapy.
Episodes of apnea, cyanosis, and paroxysmaleye movements can occur, and
these can be preceded by convulsions. Motor impairments (ataxia,
dystonia, spastic disorders) are then added in, and microcephaly forms.
The EEG often shows generalized or local epileptiform
changes. An important pathognomonic feature of the disease is the
regression of epileptic seizures and EEG anomalies after ingestion of
food. The non-epileptic variant is dominated by motor disorders:
paroxysmal dyskinesias (choreoathetosis/ dystonia), ataxia, and
alternating hemiplegia of different grades of severity.
Patients frequently complain of headache. In some
cases, hemolytic anemia forms part of the syndrome. Increases in
clinical symptomatology during periods of hyperthermia and addition of
inter current diseases are typical. All patients with GLUT1 experience
progressive general developmental delay The intellect is affected, as
are verbal functions (dysarthria), increasing mental delay and changes
in the motor domain.
The different variants of the disease and the
severities of the various symptoms in each individual case cause
significant difficulty in diagnosing GLUT1. Previously, the disease was
diagnosed on the basis of the clinical picture and the results of
laboratory studies, primarily - the assessment of glucose content in the
cerebrospinal fluid (CSF).
With GLUT1, a decrease in glucose concentration is
detected in CSF at normal or low lactate values against normoglycemia.
The diagnostic criterion of the disease is a decrease in glucose content
below 60mg/dl (<40mg/dl in > 90% of patients, 4152mg/dl in ~ 10%
of patients). Currently, the final diagnosis is set after a genetic
examination (DNA diagnostics).
Analysis of 3-O-methyl-D-glucose absorption in
erythrocytes (35%-74% of the standard) is currently considered as the
diagnostic gold standard for this disease [4].
SLC2A1 is the only gene where mutations are associated with the
development of GLUT1 deficiency syndrome. SLC2A1 gene encoding GLUT1
protein, consists of 10 exons and 9 introns, is localized on the short
arm of chromosome 1 (1p34.2) [5]. More than 150 mutations in SLC2A1 gene, which are the cause of GLUT1 deficiency syndrome, are described [6].
Pathogenic variants are represented by missense, nonsense mutations,
which may include small intragenic deletions/insertions, as well as
variants of splicing sites.
Proteins are carriers of glucose from GLUT protein
group. These transport proteins facilitate passive diffusion of glucose
through tissue seals by means of energy-independent mechanisms. The
group includes 12 GLUT proteins. GLUT1 is expressed in endothelial cells
of blood vessels that form part of the blood-brain barrier and is
responsible for the penetration of glucose into the brain. GLUT2 is
associated with the FanconiBickel syndrome, GLUT3 is responsible for the
penetration of glucose through the neuronal plasma membrane, GLUT4 is
an insulin-regulating glucose transporter of adipose tissue, cardiac
muscle and skeletal muscles, and is responsible for insulin-mediated
glucose transport, GLUT5 is expressed in the intestines, testicles and
kidneys. The function of GLUT7 is currently unknown [7-9].
Currently the only effective approach to the
treatment of GLUT1 consists of a ketogenic diet (KD). Consumption of a
high- fat, low-carbohydrate, ketogenic diet is accompanied by the
formation of ketone bodies, which are able to cross the blood- brain
barrier using the MCT-1 transporter, supporting alternative energy
metabolism in the CNS [10-14].
The KD method was developed earlier for the treatment
of drug-resistant epilepsy. In the Russian Federation, the only center
that applies KD for the treatment of non-curable epilepsy is the State
Budgetary Health Care Institution Scientific and Practical Center for
Specialized Medical Care for Children n.a. V.F. Voyno-Yasenets Health
Care Department of Moscow.” In 2010, we have received a patent for
invention No. 2404777 "A method for treating pharmaco-resistant
epilepsy”
Published data and our own results [8-17]
show that the use of a KD in epilepsy leads to improvements, with
decreases in the frequency of seizures by 50-75% in more than half the
patients and complete termination of seizures in 18%. In practice,
various modifications of the KD are used depending on the child's age
and individual characteristics.
The KD is characterized by a high content of fat,
which leads to metabolic acidosis, which can elicit side effects such as
dyslipidemia, osteopenia, biliary dysfunction, gastroesophageal reflux,
constipation, diarrhea, hyperuraturia, cardiopathy, etc. [10,11,13,17].
The phenomenon of aggravation of seizures has been described, along
with changes in the emotional background (disinhibition, irritability) [12].
Patients on a KD therefore require careful
observation for maintenance of the therapeutic level of ketosis and
appropriate prophylaxis and correction of possible complications [15-18]. A KD was first used in GLUT1 in 1991. As evidenced by published data [2,19],
use of a KD in GLUT1 allows convulsive states to be eliminated and
provides improvements in motor and cognitive functions and metabolic
parameters, and, if prescribed early, improvements in the long-term
neurological outcome [3].
According to the published data, 95% of children with
seizures with GLUT1 with KD showed a reduction in seizures by more than
50%, and 80% - a reduction in seizures by more than 90% [20].
Many centers that treat GLUT1 give recommendations on the use of
classical KD with a high ketogenic ratio of 4:1 and control the increase
in ketones in blood serum or in urine, which is confirmed by our
clinical data [20],
but there are other data showing that there is no differences between
patients using KD with a ratio of 4:1 (more strict) and lower ratios. 5
of 16 (31%) patients adhering to a ketogenic ratio of 4:1 using KD were
free of seizures compared with 21 of 38 (55%) for lower ketogenic ratios
[20].
Antiepileptic drugs (AED) are generally ineffective
inGLUT1, or have only limited application, and some are contraindicated.
This relates to barbiturates, which are often used in children in the
first year of life, as well as valproates, acetazolamide, topiramate and
zonisamide [21]. Treatment with methylxanthines should be avoided.
Alternative therapies are being developed recognizing
that side effects occur in children with GLUT1 with prolonged use of
KD. They include a modified Atkins diet (MDA) [22-25], ketoesters [26], triheptanone [27], alpha-lipoic acid [28] and acetazolamide [29].
In the southeast medical center of the University of
Texas in Dallas, Dr. Juan M. Pascual conducts a number of new studies
using triheptanon-C7 edible oil in respect of GLUT1. The ultimate goal
of the study using the proposed C7 diet is to answer the question of
whether C7 influences the effectiveness of neuropsychological activity
(cognitive abilities) in patients with GLUT1 who observe and do not
observe KD. There is a concern that C7 may have a negative effect on KD
and, as a result, researchers intend to thoroughly study the potential
compatibility/incompatibility.
Triheptanon (C7) is a food product considered as a
possible therapeutic food. Perhaps, C7 will soon appear in the market as
a therapeutic food along with other widely available food additives,
such as vitamins (NANO VM) or MCT oil [30].
Research Objective
To increase the level of diagnostics of patients with
glucose transporter type 1 deficiency syndrome (GLUT1) and to give
recommendations on their treatment using KD.
Subject of Research
In the neuropsychiatric department of the State
Budgetary Health Care Institution Scientific and Practical Center for
Specialized Medical Care for Children n.a. V.F. Voyno-Yasenets Health
Care Department of Moscow, patients with confirmed glucose transporter
type 1 deficiency syndrome (De Vivo disease) associated with mutations
in SLC2A1 gene have been observed for 6 years. The permission of the
Ethical Committee of the Scientific and Practical Center for Specialized
Medical Care for Children was obtained after the parents signed
voluntary informed consent.
Methods of Research
Molecular-genetic research was conducted using modern
diagnostic methods. The diagnosis of GLUT1 deficiency syndrome in the
first examined patient was confirmed by method of targeted exome
sequencing of the panel of 34 genes associated with early forms of
epileptic encephalopathy, first developed in the genetic laboratory of
the SPC of [31].
Isolation of genomic DNA from peripheral blood leukocytes and
subsequent targeted exome sequencing was performed on MagNA Pure LC 2.0
and 454 Sequencing GS Junior (Roche) analyzers, respectively.
Specialized Internet resources were used to predict the conservatism and
the degree of pathogenicity, clarifying the clinical significance of
all identified variants in the genes: SIFT (Predict effects of
nonsynonmous/missense variants), PolyPhen-2 (prediction of functional
effects of human nsSNPs) u MutationTaster.
Samples of "1,000 Genomes”, ESP6500, Exome
Aggregation Consortium (ExAc) and Genome Aggregation Database (gnom AD)
were used to estimate the population frequencies of the identified
variants. The OMIM database, specialized databases (GeneReviews (NCBI),
HGMD (The Human Gene Mutation Database), LOVD (Leiden Open Variation
Database), ClinVar (NCBI) and published data were used to assess the
clinical relevance of the identified variants.
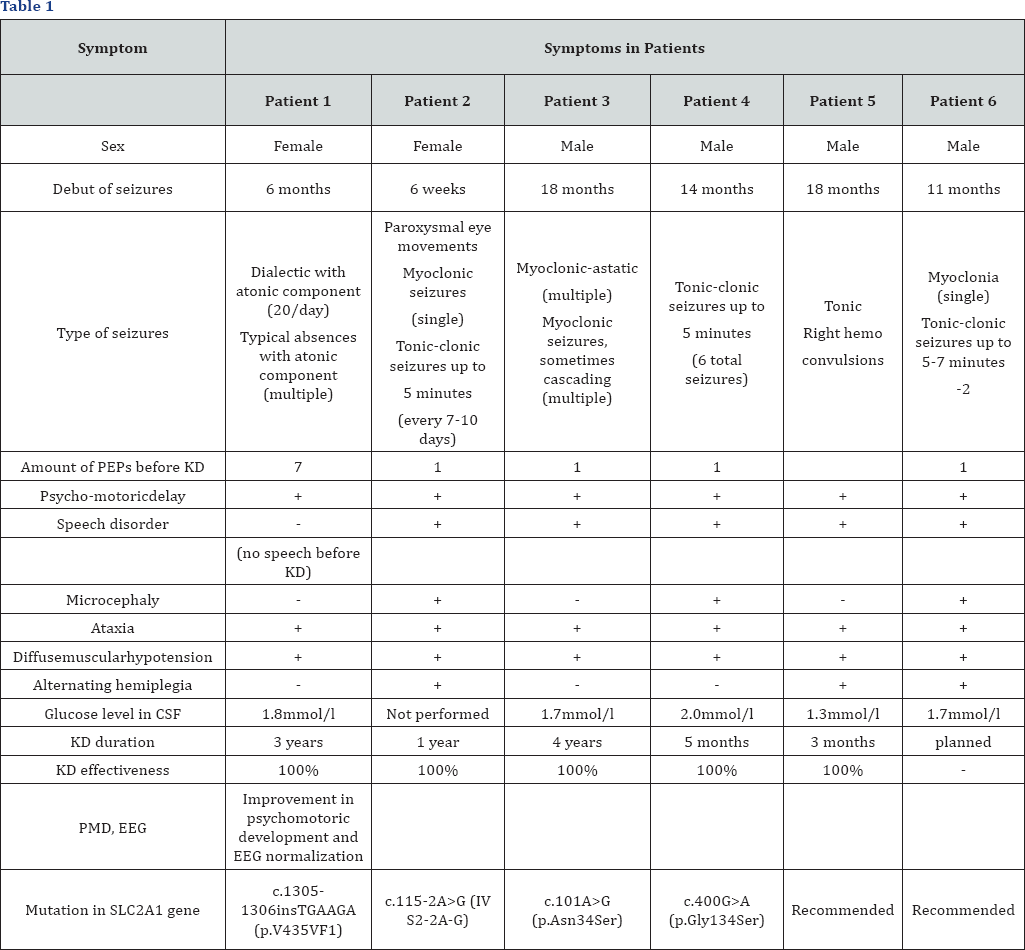
The detected mutation in SLC2A1 gene is represented
by insertion of additional 6 nucleotides into the sequence-c.1305-
1306insTGAAGA (p.V435VFI). The mutation is not registered in the control
samples of "1000 genomes”, ESP6500 and ExAC. Algorithms for predicting
pathogenicity regard this substitution as pathogenic [32].
In 2 patients, mutations in SLC2A1 gene were
determined by direct Sanger sequencing: c.115-2A>G (IV S2-2A-G)
andc.101A>G (p.Asn34Ser). When performing the exome sequencing,
mutation c.400G>A (p.Gly134Ser) was detected in 1 patient, which was
not registered in the control samples of "1000 genomes”, ESP6500 and
ExAC. Algorithms for predicting pathogenicity regard this substitution
as likely pathogenic.
The diagnosis of GLUT 1 deficiency syndrome in two
patients was based on the clinical picture, data of biochemical analysis
of the cerebrospinal fluid (a decrease in the level of glucose in CSF
below the threshold level of 2.2-3.3mmol/l) and detection of pathogenic
mutations in SLC2A1 gene. Two patients were diagnosed according to the
clinical picture and biochemical analysis of CSF, since no informed
consent was given to genetic testing.
Patients observed by us showed different types of
mutations in SLC2A1 gene. Due to the small number of observations, it is
not possible to carry out genotype-phenotypic correlations. All
children were admitted with a diagnosis of cryptogenic epilepsy, a delay
in psychomotor and speech development.
It is known from the anamnesis that all 6 patients
from full-term normal pregnancies, independent births on time, had a
good birth weight and a high APGAR scale score. The period of newborn
childhood was uneventful. However, in the future, psycho-speech
development slowed, ataxia and epileptic seizures appeared.
Part of the patients showed an increase in the
frequency of seizures during "hunger”, as well as lethargy and
drowsiness. After eating, the children's condition improved, epileptic
seizures disappeared. Further, all 6 children reported complaints of
weakness in the legs that increased after physical exertion. EEG
revealed epileptiform multiregional activity, periodically with
secondary generalization. The effect of taking anticonvulsants was
ambiguous.
Clinico-laboratory and instrumental studies showed a
decrease in the concentration of glucose in CSF to 1.3-2.0mmol/l
(2.2-3.3mmol/l); the level of glycemia in blood was 4-5mmol/l (standard
3.9-6mmol/l); the ratio of glucose in CSF to blood glucose was
0.3-0.45mmol/l (standard 0.54-0.56mmol/l). The level of lactate in blood
was increased (standard 0.5-2.2mmol/l).
EEG of some children before eating showed an
irregular α-rhythm, generalized discharges of epileptiform activity;
after eating - a regular α-rhythm, regress of epileptiform activity.
Magnetic resonance imaging (MRI) of the brain showed no pathology in all
patients. Based on the results of the studies, the following disease
was diagnosed: Glucose transporter type 1 deficiency syndrome (GLUT1).
Epilepsy
A molecular genetic examination confirmed GLUT1 in 4
patients. These children were immediately taken to pass the KD course.
The preliminary examination revealed no contraindications on the part of
the somatic and neurological statuses for using this method of therapy.
With the introduction of KD all children took AEDs in connection with
epileptic attacks: myoclonic, myoclonic-atonic, complex absences and
tonic-clonic. Often, myoclonias had a cascading behavior, intensified
and becoming more frequent in a state of hunger. The neurological status
showed motor disinhibition, expressive speech in the form of single
words, poor vocabulary, dysarthria, diffuse muscle hypotension, motoric
awkwardness, ataxia.
Results
A positive effect was observed after KD in the form
of complete relief of epileptic seizures in all patients, absence of
epileptiform activity according to video EEG monitoring data,
improvement of EEG frequency characteristics, complete abolition of
perinatal encephalopathy, however, the psycho-neurological deficiency in
the form of hypotension, discoordination and dysarthria retained, but
with expressed improvement.
Patients are being continuously monitored at home
with regular monitoring of the children's condition, as provided for in
the protocol. Symptomatic therapy was recommended to prevent and correct
side effects: preparations of pancreatic enzymes, cholagogues,
prokinetics and probiotics, as well as constant intake of
multi-vitamin-mineral complexes.
Positive changes in cognitive and speech development
have been achieved even after 3 months from the beginning of diet
therapy: socialization of children has improved, interest in viewing
television programs, surrounding subjects has increased, and phrase
speech has appeared (separate phrases and sentences). At the same time,
motoric disinhibition, restlessness, and periodical aggressiveness and
irritancy retain in some children.
In our center, we use a metabolic drug - carnitine to
improve metabolic processes, reduce manifestations of asthenic
syndrome, MCT or coconut oil to increase the level of ketosis,
gamma-aminobutyric acid and choline alphoscerate - to improve
neurocognitive functions.
Later, in all patients on the background of KD
administration and concomitant therapy, progress in cognitive and speech
development grew, as well as interest in games, learning, expressive
speech improved - simple sentences appeared, children started attending
kindergartens and auxiliary schools. Quality of life of families and
patients significantly improved.
Differential diagnostics of GLUT1 deficiency syndrome
was performed with other pathological conditions causing
neuroglycopenia (chronic or transient hypoglycemia in familial
hyperinsulinism), convulsions in newborns and microcephaly, in
particular, early manifestations of Rett syndrome, Angelmann syndrome,
infantile forms of neuronal ceroid-lipofuscinosis; opsoclonus-myoclonus
syndrome; cryptogenic epileptic encephalopathy with a delay in
development; familial epilepsy with autosomal dominant type of
inheritance; episodes of paroxysmal neurological dysfunction in response
to carbohydrate intake, especially when combined with alternating
hemiparesis, ataxia, cognitive impairment, or convulsions; motoric
disorders, including dystonia [31].
Discussion
According to Columbia University (results obtained
from many patients around the world and are similar to university
results), patients with GLUT1 receiving KD can achieve a seizure
reduction of more than 90% without using AEDs [20]. In our clinic, we managed to achieve 100% control of seizures, apparently due to a small number of patients.
According to global and our own published data, the
overall results are better among those who started diet therapy at an
earlier age. Patients diagnosed with GLUT1 in the earlier age were also
prone to achieve better results than older patients. Screening of SLC2A1
gene will help speed up early diagnostics of GLUT1 and may lead to a
faster KD appointment [20].
Alternative GLUT1treatments, such as the use of triheptanon, are the
subject of clinical research; our foreign colleagues and we are
convinced that the introduction of KD cannot be delayed.
The results of foreign and our studies contribute to
approval of dietary therapy as the "gold standard” for GLUT1 treatment;
it remains unclear what specific diet should be used. According to our
colleagues, among children without seizures, the percentage of those
observing KD and MCT (including triglycerides with an average chain
length) diet was approximately equal to the percentage of those
observing MDA and hypoglycemic diet, 74% and 63%, respectively [20].
Among the patients using classical KD, the ketogenic
ratios vary considerably from 4:1 to 2:1, and also include the MCT
diet.The results are almost identical among all diets and ratios [20].
We noticed a certain trend towards better signs of absence of seizures
and improvement of cognitive functions among those with a 4:1 ratio, but
nevertheless, this cannot reflect the modest size of our sampling.
There is a dependence of control over seizures on the level of ketones
in the serum (the higher the level). Monitoring of the comparison of
ketone levels in blood and urine can be important, especially given that
daily use of scarifiers in a sick child can be cumbersome and costly
for the family in financial terms [20].
In our center, we determine the level of ketone bodies in both serum
and urine. Two children (33%) had a clear dependence of the absence of
seizures on the level of ketone bodies. And finally, according to our
data and the data of foreign authors, all patients with GLUT1 on the
background of diet therapy should receive symptomatic therapy to improve
tolerability of KD and reduce side effects from its application, as
well as nutritional supplements and nootropic drugs to improve cognitive
functions.
Conclusion
GLUT1 deficiency syndrome associated with impaired
glucose transport to the brain, as a result of mutations in SLC2A1 gene,
leads to neurological disorders with large phenotypic variety. Spinal
puncture should be performed in each patient with suspected GLUT1
deficiency syndrome with glucose level measurement. Reduction in the
glucose concentration in CSF of less than 2.2mmol/l is an indication for
the molecular genetics study of SLC2A1 gene and early KD
administration.
Satisfactory tolerability of KD at high efficiency
(almost 100%) in respect of epileptic seizures and significant
improvement in motoric and cognitive functions is the grounds for its
continued use in these patients, especially given the fact that diet
therapy is currently the only effective GLUT1 treatment. Medico-genetic
counseling of families where the child has a confirmed GLUT1 syndrome is
important in planning the next pregnancy. The type of inheritance is
autosomal-dominant, the risk of transmitting a pathogenic mutation from
parents to the child is 50%. When a mutation in SLC2A1 gene is detected
in a proband, it is recommended to subject the parents to molecular-
genetic testing, as parents may have a subclinical form of the disease [32].
The above medical cases show availability of KD in treatment of
patients with such a rare and severe hereditary disease as glucose
transporter type 1 deficiency syndrome (GLUT1)



Comments
Post a Comment