Review ArticleVolume 1 Issue 5 - June 2017DOI:10.19080/NAPDD.2017.01.555574Nov Appro Drug Des DevCopyright © All rights are reserved by Ram Charitra MauryaBioconjugation among Metallopharmaceuticals-Open Access Publishers
Novel Approaches in Drug Designing & Development (NAPDD)
This review pertains to an effort to notify the
importance of metal binding of naturally occurring molecules.
Metallopharmaceutical science is a huge discipline of multifarious
applications. In due course of design of metallic drugs one has to rely
upon biological relevance of the compound. Sometimes the target activity
is lost into toxicity. Hence, the association of a biomolecule or
modified bio-compound coordinated with metallic system is the essence of
bioconjugation and is the need of hour. Bio-conjugated metallic
complexes are always praised for better action. Some diseases have been
exemplified in this review and a comprehensive way of presentation has
been established throughout the text.
Keywords: Bioconjugation; AD; Diabetes; Cancer; Antioxidant Introduction
Bioconjugation is a meticulous chemical strategy to
form a stable covalent link between two molecules, at least one of which
is a biomolecule. Synthesis of bioconjugates involves a variety of
challenges, ranging from the simple and nonspecific use of a fluorescent
dye marker to the complex design of antibody drug conjugates.
Antibody-drug conjugates such as Brentuximab vedotin and Gemtuzumab
ozogamicin are examples of bioconjugation, and are an active area of
research in the pharmaceutical industry [1].
A promising strategy to enable the use of metal nuclides in
antibody-targeted imaging and therapy is to design molecules that
coordinate to the metal ion and preclude its release in-vivo [2].
A necessary prerequisite of any ligand that binds a
metal to form a contrast agent is that the resulting contrast agent be
stable so as to prevent the loss of the metal and its subsequent
accumulation in the body. Other considerations include an ability to
reversibly bind water, which in turn increases it contrastability and
decreases the dose level required. This ability is clearly important
since the interaction between any two nuclear spins through space
decreases at a rate equal to the reciprocal of the distance raised to
the sixth power [3].
Hence, metals in medicine are used in organic systems
for diagnostic and treatment purposes. Inorganic elements are also
essential for organic life as cofactors in enzymes called
metalloproteins. When metals are scarce or high quantities, equilibrium
is set out of balance and must be returned to its natural state via
interventional and natural methods. Metals play a vital role in an
immense number of extensively differing biological processes. Some of
these processes are quite specific in their metal ion requirements, in
that only certain metal ions in specified oxidation states can
accomplish the necessary catalytic structural requirement (Figure 1) [4].
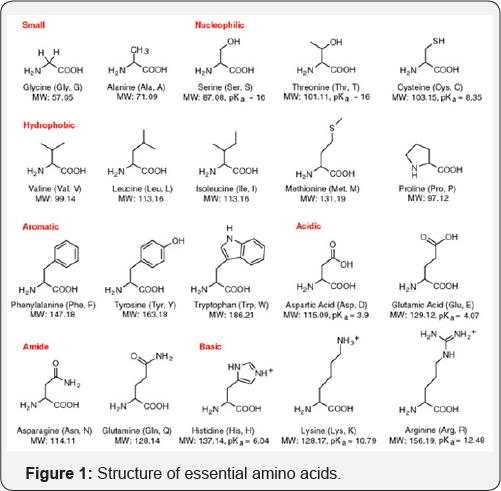
One of the principal themes of bioinorganic chemistry
is the synthesis of metal complexes that have the ability to mimic the
functional properties of natural metalloproteins [5,6].
Proteins, some vitamins and enzymes contain metal ions in their
structure involving macromolecular ligands. Inorganic and bioinorganic
chemistry are the major contributing fields of medical science and human
health witnessed by the past half century. Today, metal-containing
therapeutics constitutes a multi-billion dollar industry. Recent
investigations in bioinorganic chemistry include the use of metal ions
as synthetic scaffolds for the preparation of small molecule
therapeutics.
Insulin Mimicry via Metallic Compounds
In a continued interest towards metallopharmaceuticals (Figure 2)
Sodium vanadate and derivatives of bismaltolato- oxovanadium (IV)
complexes (BMOV) have been reported to lower levels of blood sugar in
diabetic patients [7].
In other words it may be said that scientific community is busy with
copying a hormone called as insulin to develop an ultimate treatment of
diabetes. Recent under trial experiments with Gold and Silver based
glucose level stabilizing agents have further unfurled seek for more
efficacy [8,9].
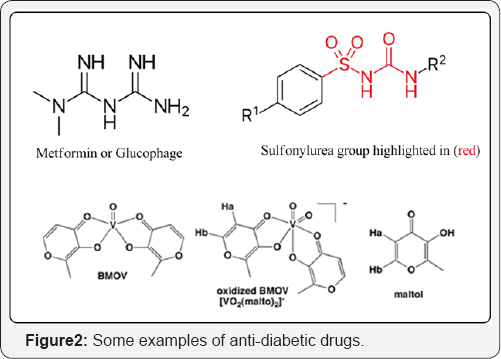
Antidiabetic drugs may be either insulin injections
which are used in serious cases of diabetes or oral hypoglycemic drugs,
and are suitable for most adult patients. Different hypoglycemic drugs
are available in market. These drugs may be classified as the following:
Sulphonylureas: increase insulin secretion and help to reduce blood
glucose levels. But sulphonylurea may cause weight gain, hypoglycemia
and allergic reactions. They are contraindicated in case of pregnancy,
lactation and diabetes type 1. They act by affecting the pancreatic
β-cells stimulates the movement of insulin-containing secretory granules
to the cell surface then into circulation. Biguanides (metformin): They
prevent production of glucose in the liver, so improve the body's
sensitivity to insulin. They may cause temporary nausea and/or diarrhea,
loss of appetite and metallic taste. They are contraindicated with
kidney or liver diseases and heart problems. Alpha Glucosidase Inhibitor
(Acarbose): They may cause diarrhea, gas, constipation, or stomach
pain. Hence, the search for more intelligent/efficient antihyperglycemic
or antihypoglycemic agents continues. Dissemination of such area of
research expects clinically approved use of metal containing compounds
for identifying new medicinal agents from throughout the periodic table
to be used as antidiabetic and antioxidant tools.
Biotransformation of Metallic Compounds
Elemental Medicine is nowadays accepted as a rapidly
developing field busy with developing novel therapeutic and diagnostic
metal complexes. Advances in biotransformation of metal complexes and
targeting, with particular reference to platinum anticancer, gold
anti-arthritic, and bismuth antiulcer drugs has remained active goal
since decades [10,11].
Studies of iron and copper complexes have shown that they can be more
active in cell destruction as well as in the inhibition of DNA
synthesis, than the uncomplexed organic ligands [12].
Hence, the field of inorganic chemistry in medicine may usefully be
divided into two main categories: firstly, ligands as drugs which target
metal ions in some form, whether free or protein- bound; and secondly,
metal-based drugs and imaging agents where the central metal ion is
usually the key feature of the mechanism of action [13,14].
In addition to metal complexes of novel ligands, compounds of metals
with already known organic pharmaceuticals like aspirin, paracetamol,
metformin, etc. have gained keen interest [15].
It has been seen that their biological relevance increases on
complexing with the respective ligands (organic medicinal chelates).
Research has shown significant progress in utilization of transition
metal complexes as drugs to treat several human diseases like
carcinomas, lymphomas, infection control, anti-inflammatory, diabetes,
and neurological disorders [16].
Cancer is the second most frequent cause of death in
the world. The discovery of antitumor activity of cisplatin began a
search for other metal complexes with cytotoxic properties against
cancer cells [17].
The instant information regarding anticancer activities of the ten most
active metals: arsenic, antimony, bismuth, gold, vanadium, iron,
rhodium, titanium, gallium and platinum have been already updated.
Despite the efficacy of cancer treatment using cisplatin, the use is
still limited due to severe side effects such as neuro-, hepato- and
nephro-toxicity and by resistance phenomena [18].
Gold (III)-dithiocarbamato complexes have recently gained increasing
attention as potential anticancer agents because of their strong tumor
cell growth- inhibitory effects, generally achieved by exploiting
non-cisplatin- like mechanisms of action [19].
The potential applications of Mo-based complexes in
medicinal chemistry as metallopharmaceuticals in treating diseases such
as cancer and tumors [20]
indicate the emphasis of significant approach of non-platin anticancer
agents. Ruthenium compounds are highly regarded as potential drug
candidates. The compounds offer the potential of reduced toxicity and
can be tolerated in-vivo. The various oxidation states, different
mechanism of action, and the ligand substitution kinetics of ruthenium
compounds give them advantages over platinum- based complexes, thereby
making them suitable for use in with promising cytotoxic profiles [21].
The role of transition metals as micronutrients as well as co-factors
of several metallo- enzymes in living systems further corroborates the
rationale behind synthesis and evaluation of novel transition-metal
based complexes for their anticancer effects [22].
Future use of substituted organic ligands and their metal complexes
would hence bring forth effective anticancer agents and would depend on
structural modifications as would afford them better potency against a
number of tumors/cancers, together with low toxicity and better
solubility.
Antioxidant Properties of Metal Complexes
An antioxidant is a molecule that inhibits the
oxidation of other molecules. Oxidation is a chemical reaction that can
produce free radicals, leading to chain reactions that may damage cells.
Antioxidants terminate these chain reactions. Transition metal
complexes have been shown to possess encouraging antioxidant activities [23].
Co(II), Ni(II), Cu(II) and Mn(II) complexes of
6-bromo-3-(3-(4-chlorophenyl)acryloyl)-2H-chromen-2-one have been
recently found to be effective antioxidants [24]. Generally, antioxidant activity of complexes are determined in- vitro by the hydroxyl radical scavenging, DPPH, NO and reducing power methods [25].
The chemical principles of methods based on biological oxidants
comprise superoxide radicals scavenging (O2•-); hydroxyl radical
scavenging (HO.); hydrogen peroxide scavenging (H2O2); peroxyl radical
scavenging (ROO.) and nitric oxide scavenging (NO.) [26].
Among the non-biological testing scavenging of 2,
2-diphenyl-1-picrylhydrazyl radical (DPPH« assay) and scavenging of 2,
2-azinobis-(3-ethylbenzothiazoline- 6-sulphonate) radical cation (ABTS
assay) are mostly experimented. Furthermore, thiobarbituric acid
reactive substances (TBARS) and protein carbonyl assays have also been
the subject of great attention in this context [27,28].
The novel electrochemical approach to antioxidant activity assay based
on the reaction with stable radical 2,2'-diphenyl-1-picrylhydrazyl
(DPPH) monitored by the rotating disk electrode (RDE) method has been
described advantageous in comparison with usual spectrophotometrical
assay since it can be applied to colored compounds and in a wide range
of concentrations [29].
Dementia Relevant Metallic Systems
Alzheimer's disease currently affects over 5.4
million Americans with $236 billion spent annually on the direct costs
of patient care [30].
Studies on antioxidant drugs would surely open successful doors to
treat AD patients. Seeking for potential antioxidants, chemical behavior
of Quercetin as antioxidant and metal chelator has become the subject
of intense experimental research [31].
Under comparative antioxidant studies of Co(II), Ni(II), Cu(II) and
Mn(II) complexes of 6-bromo-3-(3- (4-chlorophenyl)acryloyl)-2H-
chromen-2-one Ni(II) complex shows superior antioxidant activity than
other complexes [32]. Commonly it is has been observed that metal complexes may serve as better free radical scavengers [33-35]
as compared to the respective free ligands. In some cases antioxidant
complexes have rendered a well pronounced larvicidal activity [36].
Hence, synthetic chemistry is playing revolutionary role in human
beings by synthesizing novel compounds by different techniques [37].
The target of scientific community has been thus to prepare bioactive
compounds relevant to anticancer, antioxidant and enzyme inhibition
studies at both the in-vitro as well as in-vivo fronts.
Biomarkers
Biochemical pathways are famously complex and
interconnected, so it’s no surprise that depictions of them have to be
simplified (Figure 3).
Increasingly, molecular and cell biologists have been coming to terms
with the fact that it is hard to decide a label for some protein as a
green fluorescent protein (GFP) and expect it to carry on as before.
Putting a star next to its name on the whiteboard, or renaming it
‘Target-GFP’, doesn't capture what’s really going on. It is very, very
hard to observe living systems at the molecular level without perturbing
the very things trying to see, but a great deal of effort is now going
into trying to minimize these effects [38].
Under the light shed for evaluation of antidiabetic and antioxidant
research, besides developing biomarkers treatment strategies have also
been the subject of huge interest. The current status of the aimed field
in terms of literature survey is discussed below: (Figure 3).
Diabetes and Bio-Conjugation
With the aim to continue the enthusiastic search of metallopharmaceutical drugs against diabetes [39,40],
thiazolidinediones (TZD) have been reported to be effective
anti-diabetic agents that improve insulin sensitivity through the
activation of the nuclear receptor and adipocyte-specific transcription
factor, peroxisome proliferator-activated receptor gamma (PPAR-γ) [41].
Recently it has been found that Selective PPARγ modulators (sPPARγM)
retain insulin sensitizing activity but with minimal side effects
compared to traditional TZDs agents [42].
A combination of virtual docking, Surface plasmon resonance (SPR)-based
binding, luciferase reporter and adipogenesis assays have been
suggested to enlighten the interaction mode, affinity and agonistic
activity of L312 to PPARγ in-vitro, respectively [43].
The pharmaceutical isoforms having anti-diabetic effect act by
improving the biochemical parameters, this effect is probably due to the
high content of polyphenolic compounds found in the formulations [44].
In due course of finding a successful
antihyperglycemic candidate, metallic compounds like Vanadium complexes
have been well demonstrated in streptozotocin-induced (STZ) diabetic
rats and was found that that the vanadate and vanadyl forms of vanadium
possessed a number of insulin-like effects in various cells [45].
In the current times basic aspect of diabetes including insulin
molecular characterization, chemical basis and its secretion,
hypoglycemic drugs and their mode of action associated with diabetes are
among the main quests being searched [46].
In an approach of comparative antidiabetic studies of isoforms of BMOV
having different metallic centres, it has been found that none so far
has surpassed bis (maltolato) oxovanadium (IV) (BMOV) for glucose- and
lipid-lowering in an orally available formulation [47]. It is hence clear that ligand and metal selection should be meticulously done to formulate efficient antidiabetic compound.
The bioconjugate chemistry of antihyperglycemic
metallic complexes have presented worth some results. The conspicuous
application of chromium (III)-amino acid complex against
nicotinamide-streptozotocin induced diabetic Wistar rats showed that
supplementation of Cr(III)-complex in 8 weeks decreased the blood
glucose level in range 46.446-79.593% [48]. Similarly, vanadyl (IV) adenine complex has been introduced as a new drug model for the diabetic complications [49].
Therefore it is expected to be worthy if derivatives of biogenic
ligands are formed to design a ligand of favourable properties. For
instance, zinc metal-organic framework (MOF) synthesized under mild
hydrothermal routes using 5-aminotetrazole and methyl-2-
amino-4-isonicotinate anionic ligands has been reported to possess a
well pronounced in-vivo antidiabetic activity and low in-vitro cell toxicity [50]. With the same effort,
N,N-Dimethylbiguanide hydrochloride complexes of
Neodymium introduced as oral glucose-lowering agent to treat non-insulin
dependent diabetes mellitus and to act as antioxidant has shown
prominent effect of functional group position in the respective ligands [51].
The medical properties of naturally occurring
compounds such as chromones, flavonoids and coumarins are expected to
enhance when complex with metal ions suggest the importance of
bioconjugate chemical drug research. These complexes can be successfully
used in the satisfactory treatment of diseases such as diabetes
mellitus [52].
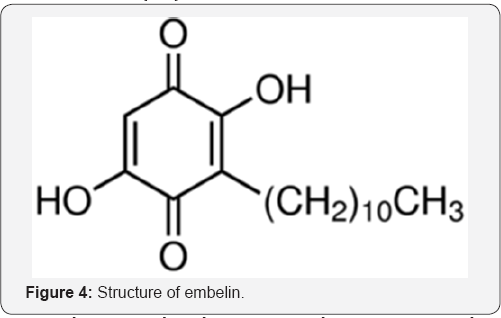
In recent years regulation of the enzymatic activity of human aldose
reductase (HAR) has been the main focus of investigation, due to its
potential therapeutic application in Diabetes mellitus (DM). Docking
behaviour of human aldose reductase (HAR) with different ligands namely
such as embelin (Figure 4),
copper-embelin complex, zinc- embelin complex, vilangin and quercetin
evaluated along with their putative binding sites using Discovery Studio
Version 3.1 has shown that that vilangin has maximum interaction energy
(-48.94kcal/mol) and metformin with the least interaction energy
(19.52kcal/mol) as compared to the other investigated ligands [53].
Therefore, it is strongly suggested that such type of study outcomes
might provide new insight in understanding these seven ligands, as
potential candidates for human aldose reductase (HAR) inhibitory
activity & for the prevention of Diabetes mellitus (DM) associate
disorders.
Based on combined in-vitro and in-vitro antioxident evaluation of resveratrol (Figure 5)
and molecular modeling studies, it has been indicated that
ligand-target interactions/ biological activities are largely dependent
on enantiomerism of a target compound [54].
Antioxidant Activity and Bioconjugation
Antioxidant studies are carried out at the cost of various standard methods [55].
Metal dyshomeostasis is known to be linked with numerous diseases such
as Alzheimer's and Parkinson’s diseases, cancer, etc. Recent studies
have indicated that some of the metallic compounds of certain ligands
may be active while some render inactivity when antioxidant activity
test was carried out using picryhydrazyl (DPPH) [56]. On one hand polyphenols have been suggested as efficient antioxidant and anti inflammatory candidates [57]
and on the other hand their metallic compounds are expected to exhibit
enhanced antioxidant activity due to flexible oxidation state of a
metallic centre [58].
Nickel complex of the non-steroidal antiinflammatory drug diflunisal
(Hdifl) resulted in the additive antioxidant effect of the respective
ligand [59].
The antioxidant activity of the ligand,
bis(N-(3-methoxy- salicylidene)-4-amino -phenyl)ether (H2L) and its
metal complexes Mn(III) and Cu(II) complexes determined by DPPH,
superoxide, hydroxyl and ABTS radical scavenging methods in- vitro,
suggest that the Cu(II) complex exhibits greater antioxidant activity
against DPPH, superoxide, hydroxyl and ABTS radicals than those of the
ligand and the Mn(III) complex [60].
The biotin- 8-hydroxyquinoline conjugates and their metal complexes
with manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II) have
also been well studied for the possible application in oxidative stress
[61]. Similar fashion has been observed with the metallic compounds of
p-coumaric acid [62], 2-(3-amino-4, 6-dimethyl-1Hpyrazolo[ 3,4-b]pyridin-1-yl)aceto-hydrazide [63], chromone Schiff base (Figure 6) [64], etc.
Another important aspect of the antioxidant studies
is the strength of a complex not to undergo ROS generation to render a
mechanistic action without harming a normal mammalian cell e.g., Ag
complex of 1, 10-phenanthroline [65] has shown an interesting behaviour in this context.
Sugar and Urea Derivative Based Complexes
Urea derivatives bonding through the nitrogen, sulfur
and oxygen atoms to the central metal ion form an important class of
biologically active ligands. They have been receiving considerable
attention due to their pharmacological properties, anti tubercular
activity, antiviral potentiality, activity against protozoa small pox
and certain kinds of tumour [66].
The chelating characters of thiosemicarbazone have been studied very
widely with different metal ions, their complexes with transition and
non transition elements were reported.
The ability of sugars to sequester metals is of
current interest in the possible development of metal chelates for
clinical use and as models for biologically important compounds. Amino
sugars form Schiff base with salicylaldehyde and other aromatic
aldehydes and only few reports of transition metal complexes of these
ligands have been found. Metal chelation could be a rational therapeutic
approach for interdicting Alzheimer's disease (AD) pathogenesis.
Amyloid plaques that are clusters of proteins and metal ions accumulated
between neurons (nerve cells) in Alzheimer's patients’ brains.
Enhancing the targeting and efficacy of metal-ion chelating agents
through sugar appended ligand is a recent strategy in the development of
the next generation of metal chelators.
Conclusion
From the overall survey it has been established that
biomolecules impart great effects in metallic systems to develop
molecules of interest. Metallopharmaceuticals are engaged in designing
heme-oxygenase and nitric oxide synthase models to bring forth highly
demanded gasotransmitter efficiency applicable at various bio-essential
routes. Under these circumstances scientific community should fabricate
bioconjugated systems to form compounds of human beneficial and
multi-purposeful.


Comments
Post a Comment