Tumor Metabolism and Neuroimaging: Proton Magnetic Resonance Spectroscopy in Cerebral Glioma-Juniper Publishers
Global Journal of Intellectual & Developmental Disabilities (GJIDD)
The advent of nuclear neuroimaging techniques has
vastly changed neurobiology and neuroscience from a bench-research
oriented practice to a clinical sub discipline with diagnostic and
therapeutic implications. In particular, magnetic resonance spectroscopy
(MR spectroscopy) began as a tool in understanding cellular metabolism
in tissues of the prostate gland, kidney, and brain, and has since
evolved into a useful diagnostic and prognostic tool in clinical
neurology [1]. In this review, the use of proton MR spectroscopy in the diagnosis and grading of glioma is summarized.
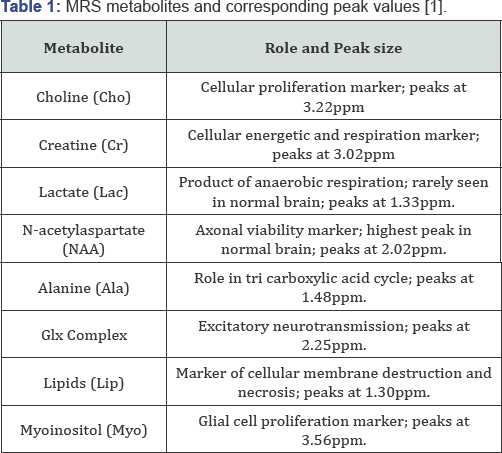
Keywords: Glioma; Magnetic resonance spectroscopy; Neuroimaging; MetabolismAbbreviations: MRS: Magnetic Resonance Spectroscopy; NMR: Nuclear Magnetic Resonance; PMRS/HMRS: Proton Magnetic Resonance Spectroscopy.
Introduction
Magnetic resonance spectroscopy, abbreviated MRS is a
biochemical technique used to identify the concentration of various
cellular metabolites in tissue. The origins of MRS lie within the
physical sub discipline of nuclear magnetic resonance spectroscopy
(NMR), which was used as early as the 1950's for identification of
nuclear magnetic moments of nuclei. Since the introduction of NMR to
clinical medicine in the form of magnetic resonance imaging (MRI), many
improvements have been made to quantify metabolite concentrations rather
than just to provide anatomical images. In particular, proton magnetic
resonance spectroscopy (proton MRS or 1HMRS) was developed as an
accurate and highly sensitive neuroimaging technique to assess metabolic
changes in brain tumors and other degenerative central nervous system
diseases.
Proton MRS technology is based on the chemical shift of hydrogen atoms in targeted tissues, according to the Larmor equation [1,2]. f= ϒHB0.
where the resonance of the hydrogen nuclei, f, is equal to the product
of the gyro magnetic ratio constant of the species (in this case 1H), ϒH,
and the external magnetic field applied by the MRS apparatus, B0.
Interactions between the hydrogen atoms and surrounding particles
produces the chemical shift: a change in magnetic field, thereby
emanating a frequency within the MRS spectrum. Frequencies can be
measured in parts-per-million, and are shown on the x-axis of an MRS
graphic plot. The vertical, y-axis of an MRS plot is the relative
magnetic field strength, or amplitude generated by a particular
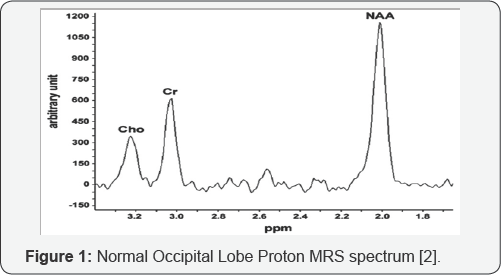
metabolite, See Figure 1. Metabolite with normal spectra peaks are listed in Table 1.
Along with anatomical imaging provided by MRI, proton
MRS has been shown to have value in the characterization, preoperative
grading, and prognosis of cerebral gliomas [3].
Whereas conventional imaging is especially adept at identifying tumor
location, size, and hemorrhage, proton MRS can aid in further evaluating
the cellular metabolism of the tumoral microenvironment and thereby
present insight into the invasiveness of such neoplasm. The biochemistry
and molecular physiology of the tumor and peritumoral areas can be
evaluated for extent of micro necrosis and gross cell differentiation,
and can be useful in cancer grading and prognostic outlook calculations [3,4].
It is agreed on, and accepted in the literature that
the hallmark of many cerebral neoplasms have the following conglomerate
of metabolite levels on proton MRS imaging [2-9]:
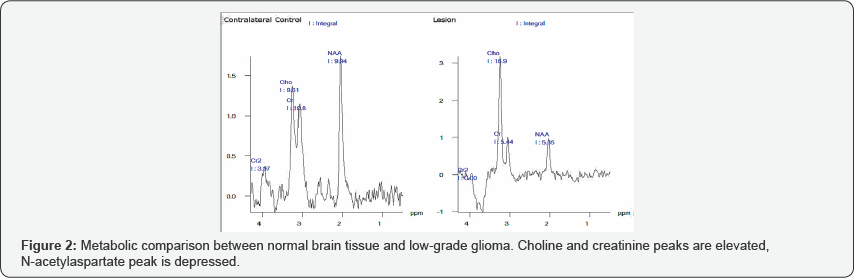
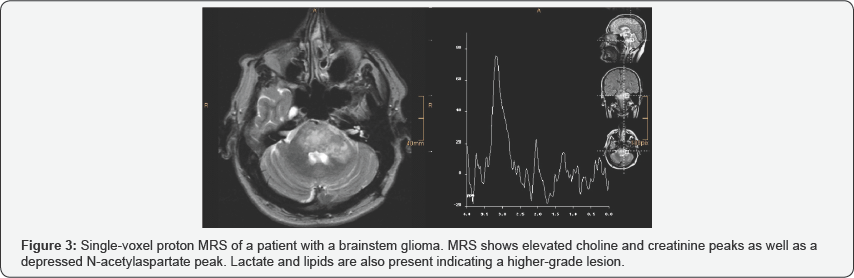
i. N-acetylaspartate (NAA) decrease, secondary to loss of axonal viability.
ii. Choline (Cho) increase, secondary to neo plastic cell proliferation.
iii. Lipid (Lip) increase, secondary to peritumoral
necrosis (if lesion is of a higher grade).In differentiating between low
and high-grade gliomas metabolites such as choline, Glx complex and
myoinositol can be used [1,3-5]:
i. Choline (Cho) peak heights are generally proportional to aggressiveness of a glioma.
ii. High myoinositol peak height generally corresponds to higher-grade dysplastic or anaplastic glioma.
iii. High Glx complex (glutamine, glutamate, and GABA) peak height corresponds to a higher-grade glioma.
Discussion and Conclusion
In addition to the grading of a cerebral glioma,
proton MRS is a useful tool in delineating tumoral margins
non-invasively. In areas around a given neoplasm, higher proton MRS
peaks of choline and Glx-complex indicate positive margins, information
which is crucial for neurosurgeons in the operating room. Overall, the
adjuvant employment of proton MRS in glioma evaluation, along with
conventional neuroimaging and neuropathology increases physician
awareness, and improves subsequent quality of care. Case-examples of
proton MRS use in glioma are shown in Figures 2 & 3.



Comments
Post a Comment